NEW DATA DEMONSTRATE QLOSI™ IS PUPIL SELECTIVE WITH CILIARY MUSCLE MOVEMENT NO DIFFERENT THAN A BALANCED SALT SOLUTION (BSS) CONTROL
SOURCE Orasis Pharmaceuticals
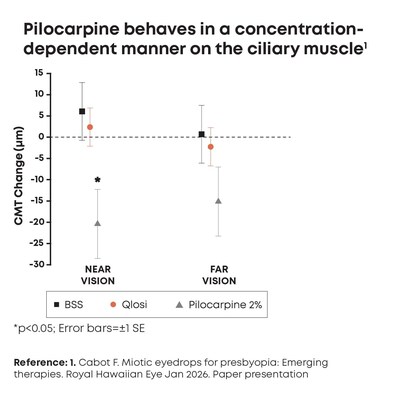
In an imaging study from the Bascom Palmer Eye Institute, Qlosi's low-concentration formulation with 0.4% pilocarpine significantly reduced pupil diameter while demonstrating ciliary muscle movement the same as BSS control1
Findings demonstrate Qlosi is pupil selective,1 establishing for the first time this unique attribute of low-concentration pilocarpine, and its ability to support presbyopia patients' near vision demands with minimal ciliary muscle response
PONTE VEDRA, Fla., Jan. 27, 2026 /PRNewswire/ -- Orasis Pharmaceuticals, an ophthalmic pharmaceutical company delivering solutions to reshape vision possibilities, announced data supporting Qlosi™ (pilocarpine hydrochloride ophthalmic solution) 0.4% is pupil selective, significantly reducing pupil diameter without a statistically significant change in ciliary muscle response in presbyopic eyes.1 These data were presented at The Hawaiian Eye & Retina 2026 Conference, held January 17-23 on the Big Island of Hawaii.
"The perception of pilocarpine historically has been based on experience with, and research conducted on, higher concentrations of pilocarpine (1% and above). Recently, the notion of a 'pupil selective' mechanism of action (MOA) has been a topic of interest," said Elad Kedar, Chief Executive Office of Orasis Pharmaceuticals. "Qlosi demonstrating minimal ciliary muscle movement, the same as the control tested, is consistent with the results we have seen in real world experience to date-very low rates of adverse events overall without a single serious adverse event reported since launch. We believe this further illustrates why formulation matters and why the concentration-dependent effects demonstrated by Qlosi reinforce how our product was designed with the safety of the presbyopia patient in mind."
The prospective, head-to-head clinical study, conducted by the Bascom Palmer Eye Institute of the University of Miami Miller School of Medicine, evaluated thousands of high-resolution optical coherence tomography (OCT) images to measure how balanced salt solution (BSS), Qlosi (0.4% pilocarpine) and pilocarpine 2%, responded to presbyopia patients' vision demands by looking at ciliary muscle movement, changes in pupil diameter and lens thickness.1 Ten subjects (mean age 51.5 years, range 46.3–64.3) were enrolled with spherical equivalent -4.00D to +2.00D.1 Exclusion criteria were prior ocular surgery or comorbidities.1
The study findings showed Qlosi significantly reduced pupil diameter but produced no significant changes in lens thickness or ciliary muscle response, compared with BSS (p<0.05).1 Pilocarpine 2% showed a statistically significant change in ciliary muscle movement at near not observed in the BSS and Qlosi groups.1
"In this study, pilocarpine 0.4% was indistinguishable from the BSS control, while the high-concentration miotic (pilocarpine 2.0%) triggered statistically significant movement of the ciliary muscle at near,1" said Florence Cabot, MD, Assistant Professor of Clinical Ophthalmology, Bascom Palmer Eye Institute, Miami, FL. "These findings are very encouraging, supporting that pilocarpine 0.4% is pupil selective and behaves in a concentration-dependent manner, reinforcing that low-concentration options may be particularly meaningful for patients considering a presbyopia therapy that balances both efficacy and safety."
In pivotal Phase 3 clinical trials, Qlosi demonstrated a favorable safety profile, with no serious side effects reported. In real-world use of Qlosi in the first 10 months of launch, there have been no reported cases of retinal detachment, or serious adverse events.2
About Presbyopia
Presbyopia is the loss of ability to focus on near objects as a result of the natural aging process. It occurs mostly after the age of 40 when the crystalline lens of the eye gradually stiffens and loses flexibility.3 There are almost two billion people globally and more than 128 million people in the U.S. living with presbyopia.3,4 People with presbyopia experience blurred vision when performing daily tasks that require near visual acuity, such as reading a book, a restaurant menu or messages on a smartphone. Presbyopia cannot be prevented or reversed, and it continues to progress gradually. Many existing treatment options can be either cumbersome or invasive, presenting a significant unmet need for presbyopia patients. Presbyopia can be diagnosed during an eye exam conducted by an eye care professional.5
About Qlosi
Qlosi™ (pilocarpine hydrochloride ophthalmic solution) 0.4% is a novel, corrective prescription eye drop indicated for the treatment of presbyopia in adults. Qlosi's EyeQ Formulation™ delivers the lowest effective concentration of pilocarpine approved in a preservative-free eye drop, with a near-neutral pH and dual lubricating agents to provide enhanced safety and patient comfort without compromising on efficacy.6,7,8 Qlosi improves near visual acuity by pupil modulation, resulting in a "pinhole effect" and an increase in the depth of field, thus increasing the ability to focus on near objects without negatively impacting distance vision. The most commonly reported treatment-related adverse events (TRAEs) were headache and instillation site pain, at rates of 6.8% and 5.8%, respectively. For more information, visit www.QlosiECP.com and follow us on Facebook and Instagram.
About Orasis Pharmaceuticals
Orasis Pharmaceuticals is an ophthalmic pharmaceutical company delivering solutions to reshape vision possibilities. Orasis has developed Qlosi™ (pilocarpine hydrochloride ophthalmic solution) 0.4%, a novel, corrective prescription eye drop for the treatment of presbyopia, or age-related blurry near vision, in adults. Orasis is led by a collaborative team of industry executives and eye care professionals working to transform the standard of care for near vision correction. Orasis is funded by a diverse group of sophisticated and experienced life science and healthcare investors. Orasis has offices in the United States and Israel. For more information, visit www.orasis-pharma.com or call 1-844-MY-ORASIS and follow us on LinkedIn.
Qlosi Indication and Important Safety Information
Indication and Usage
Qlosi™ (pilocarpine hydrochloride ophthalmic solution) 0.4%, for topical ophthalmic use is a cholinergic agonist indicated for the treatment of presbyopia in adults.
Important Safety Information
CONTRAINDICATIONS
- Hypersensitivity
WARNINGS AND PRECAUTIONS
- Advise patients to not drive or operate machinery if vision is not clear (e.g., blurred vision). Exercise caution in night driving and other hazardous occupations in poor illumination.
- Rare cases of retinal detachment have been reported with miotics. Examination of the retina is advised in all patients prior to initiation of therapy. Advise patients to seek immediate medical care with sudden onset of flashes of lights, floaters or vision loss.
- Qlosi is not recommended to be used when iritis is present.
- Qlosi should not be administered while wearing contact lenses. Remove lenses prior to the installation of Qlosi and wait 10 minutes before reinsertion.
- Avoid touching the tip of the vial to the eye or any other surface.
ADVERSE REACTIONS
- The most common adverse reactions (5% to 8%) are instillation site pain and headaches.
Please see full Prescribing Information here: qlosi.com/prescribing-information
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Media Contact:
[email protected]
412-327-9499
References:
1 Manns F, Cabot F, Ruggeri M. ORASIS–Bascom Palmer Eye Institute Study: Effect of pilocarpine eye drops on ciliary muscle accommodative response; revised analysis by Gerard Smits. Presented at Hawaiian Eye & Retina 2026; January 17-23, 2026; Waikoloa Village, HI.
2 FDA Adverse Event Reporting System (FAERS). Qlosi P. Accessed January 26, 2026. https://fis.fda.gov/sense/app/95239e26-e0be-42d9-a960-9a5f7f1c25ee/sheet/6b5a135f-f451-45be-893d-20aaee34e28e/state/analysis.
3 Holden, B. A., et al. Global Vision Impairment Due to Uncorrected Presbyopia. Arch Ophthalmol. 2008;126(12):1731-1739. https://jamanetwork.com/journals/jamaophthalmology/fullarticle/420914.
4 U.S. Census Bureau. United States Census – Populations and People. Accessed January 26, 2026. https://data.census.gov/profile/United_States?g=010XX00US.
5 WebMD. Presbyopia: Symptoms, diagnosis, and treatment. Accessed January 26, 2026. https://www.webmd.com/eye-health/eye-health-presbyopia-eyes.
6 U.S. Food and Drug Administration. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. Accessed January 26, 2026. https://www.accessdata.fda.gov/scripts/cder/ob/index.cfm.
7 Qlosi [package insert]. Ponte Verda, FL. Orasis Pharmaceuticals.
8 Holland E, Karpecki P., Fingeret M., et al. Efficacy and Safety of CSF-1 (0.4% Pilocarpine Hydrochloride) in Presbyopia: Pooled Results of the NEAR Phase 3 Randomized, Clinical Trials. Clin Ther. 2024;46(2):104-113. doi:10.1016/j.clinthera.2023.12.005.

©PR Newswire. All Rights Reserved.
Information contained on this page is provided by an independent third-party content provider. XPRMedia and this Site make no warranties or representations in connection therewith. If you are affiliated with this page and would like it removed please contact [email protected]